
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
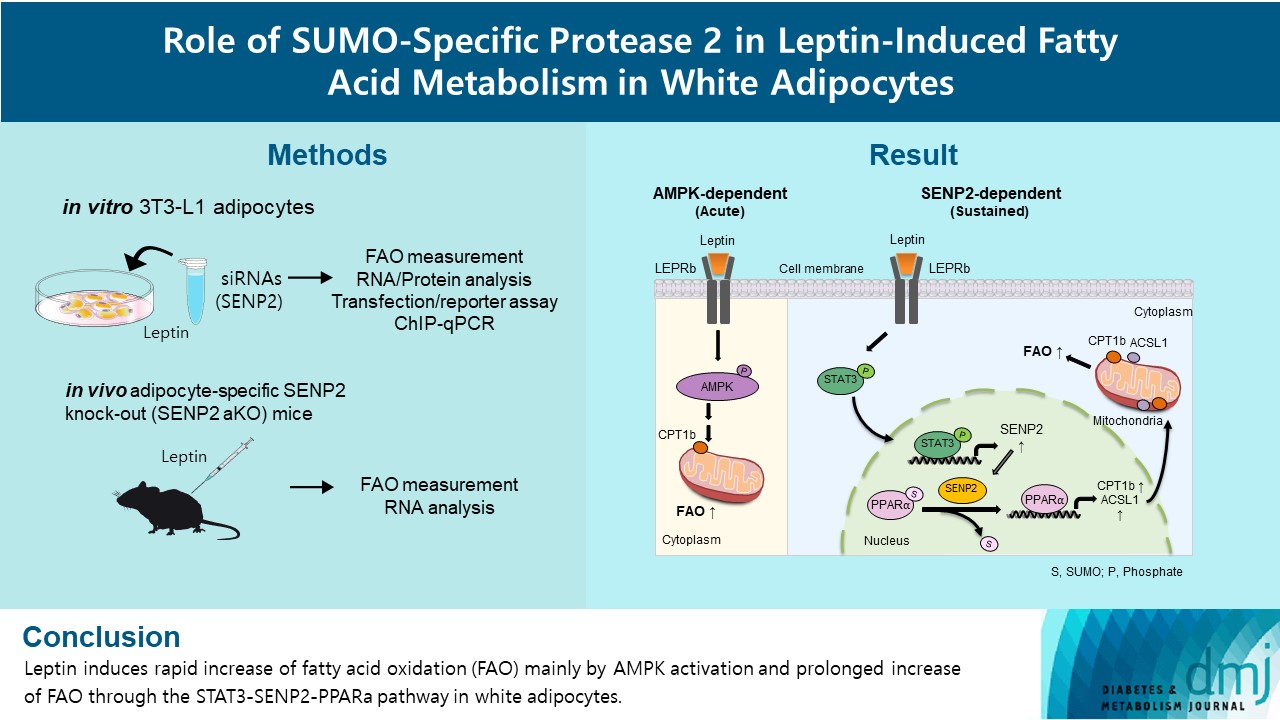
- Role of SUMO-Specific Protease 2 in Leptin-Induced Fatty Acid Metabolism in White Adipocytes
- Praise Chanmee Kim, Ji Seon Lee, Sung Soo Chung, Kyong Soo Park
- Diabetes Metab J. 2023;47(3):382-393. Published online March 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0156
- 3,223 View
- 158 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Leptin is a 16-kDa fat-derived hormone with a primary role in controlling adipose tissue levels. Leptin increases fatty acid oxidation (FAO) acutely through adenosine monophosphate-activated protein kinase (AMPK) and on delay through the SUMO-specific protease 2 (SENP2)–peroxisome proliferator-activated receptor δ/γ (PPARδ/γ) pathway in skeletal muscle. Leptin also directly increases FAO and decreases lipogenesis in adipocytes; however, the mechanism behind these effects remains unknown. Here, we investigated the role of SENP2 in the regulation of fatty acid metabolism by leptin in adipocytes and white adipose tissues.
Methods
The effects of leptin mediated by SENP2 on fatty acid metabolism were tested by siRNA-mediated knockdown in 3T3-L1 adipocytes. The role of SENP2 was confirmed in vivo using adipocyte-specific Senp2 knockout (Senp2-aKO) mice. We revealed the molecular mechanism involved in the leptin-induced transcriptional regulation of carnitine palmitoyl transferase 1b (Cpt1b) and long-chain acyl-coenzyme A synthetase 1 (Acsl1) using transfection/reporter assays and chromatin immunoprecipitation.
Results
SENP2 mediated the increased expression of FAO-associated enzymes, CPT1b and ACSL1, which peaked 24 hours after leptin treatment in adipocytes. In contrast, leptin stimulated FAO through AMPK during the initial several hours after treatment. In white adipose tissues, FAO and mRNA levels of Cpt1b and Acsl1 were increased by 2-fold 24 hours after leptin injection in control mice but not in Senp2-aKO mice. Leptin increased PPARα binding to the Cpt1b and Acsl1 promoters in adipocytes through SENP2.
Conclusion
These results suggest that the SENP2-PPARα pathway plays an important role in leptin-induced FAO in white adipocytes. -
Citations
Citations to this article as recorded by- Intermittent cold stimulation affects energy metabolism and improves stress resistance in broiler heart
Tingting Li, Haidong Wei, Shijie Zhang, Xiaotao Liu, Lu Xing, Yuanyuan Liu, Rixin Gong, Jianhong Li
Poultry Science.2024; 103(1): 103190. CrossRef
- Intermittent cold stimulation affects energy metabolism and improves stress resistance in broiler heart
- Pathophysiology
- Metformin Ameliorates Lipotoxic β-Cell Dysfunction through a Concentration-Dependent Dual Mechanism of Action
- Hong Il Kim, Ji Seon Lee, Byung Kook Kwak, Won Min Hwang, Min Joo Kim, Young-Bum Kim, Sung Soo Chung, Kyong Soo Park
- Diabetes Metab J. 2019;43(6):854-866. Published online June 27, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0179
- 6,670 View
- 115 Download
- 14 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Chronic exposure to elevated levels of free fatty acids contributes to pancreatic β-cell dysfunction. Although it is well known that metformin induces cellular energy depletion and a concomitant activation of AMP-activated protein kinase (AMPK) through inhibition of the respiratory chain, previous studies have shown inconsistent results with regard to the action of metformin on pancreatic β-cells. We therefore examined the effects of metformin on pancreatic β-cells under lipotoxic stress.
Methods NIT-1 cells and mouse islets were exposed to palmitate and treated with 0.05 and 0.5 mM metformin. Cell viability, glucose-stimulated insulin secretion, cellular adenosine triphosphate, reactive oxygen species (ROS) levels and Rho kinase (ROCK) activities were measured. The phosphorylation of AMPK was evaluated by Western blot analysis and mRNA levels of endoplasmic reticulum (ER) stress markers and NADPH oxidase (NOX) were measured by real-time quantitative polymerase chain reaction analysis.
Results We found that metformin has protective effects on palmitate-induced β-cell dysfunction. Metformin at a concentration of 0.05 mM inhibits NOX and suppresses the palmitate-induced elevation of ER stress markers and ROS levels in a AMPK-independent manner, whereas 0.5 mM metformin inhibits ROCK activity and activates AMPK.
Conclusion This study suggests that the action of metformin on β-cell lipotoxicity was implemented by different molecular pathways depending on its concentration. Metformin at a usual therapeutic dose is supposed to alleviate lipotoxic β-cell dysfunction through inhibition of oxidative stress and ER stress.
-
Citations
Citations to this article as recorded by- Metformin enhances METTL14-Mediated m6A methylation to alleviate NIT-1 cells apoptosis induced by hydrogen peroxide
Si-min Zhou, Xin-ming Yao, Yi Cheng, Yu-jie Xing, Yue Sun, Qiang Hua, Shu-jun Wan, Xiang-jian Meng
Heliyon.2024; 10(2): e24432. CrossRef - Reduced Expression Level of Protein PhosphatasePPM1EServes to Maintain Insulin Secretion in Type 2 Diabetes
Sevda Gheibi, Luis Rodrigo Cataldo, Alexander Hamilton, Mi Huang, Sebastian Kalamajski, Malin Fex, Hindrik Mulder
Diabetes.2023; 72(4): 455. CrossRef - Metformin restores prohormone processing enzymes and normalizes aberrations in secretion of proinsulin and insulin in palmitate‐exposed human islets
Quan Wen, Azazul Islam Chowdhury, Banu Aydin, Mudhir Shekha, Rasmus Stenlid, Anders Forslund, Peter Bergsten
Diabetes, Obesity and Metabolism.2023; 25(12): 3757. CrossRef - Treatment of type 2 diabetes mellitus with stem cells and antidiabetic drugs: a dualistic and future-focused approach
Priyamvada Amol Arte, Kanchanlata Tungare, Mustansir Bhori, Renitta Jobby, Jyotirmoi Aich
Human Cell.2023; 37(1): 54. CrossRef - Metformin disrupts insulin secretion, causes proapoptotic and oxidative effects in rat pancreatic beta‐cells in vitro
Maíra M.R. Valle, Eloisa Aparecida Vilas‐Boas, Camila F. Lucena, Simone A. Teixeira, Marcelo N. Muscara, Angelo R. Carpinelli
Journal of Biochemical and Molecular Toxicology.2022;[Epub] CrossRef - Protection by metformin against severe Covid-19: An in-depth mechanistic analysis
Nicolas Wiernsperger, Abdallah Al-Salameh, Bertrand Cariou, Jean-Daniel Lalau
Diabetes & Metabolism.2022; 48(4): 101359. CrossRef - Insight Into Rho Kinase Isoforms in Obesity and Energy Homeostasis
Lei Wei, Jianjian Shi
Frontiers in Endocrinology.2022;[Epub] CrossRef - Overexpression of miR-297b-5p Promotes Metformin-Mediated Protection Against Stearic Acid-Induced Senescence by Targeting Igf1r
Qingrui Zhao, Shenghan Su, Yuqing Lin, Xuebei Li, Lingfeng Dan, Yunjin Zhang, Chunxiao Yang, Xiaohan Li, Yimeng Dong, Chenchen Geng, Changhao Sun, Xia Chu, Huimin Lu
SSRN Electronic Journal .2022;[Epub] CrossRef - Metformin Dysregulates the Unfolded Protein Response and the WNT/β-Catenin Pathway in Endometrial Cancer Cells through an AMPK-Independent Mechanism
Domenico Conza, Paola Mirra, Gaetano Calì, Luigi Insabato, Francesca Fiory, Francesco Beguinot, Luca Ulianich
Cells.2021; 10(5): 1067. CrossRef - NADPH Oxidase (NOX) Targeting in Diabetes: A Special Emphasis on Pancreatic β-Cell Dysfunction
Suma Elumalai, Udayakumar Karunakaran, Jun-Sung Moon, Kyu-Chang Won
Cells.2021; 10(7): 1573. CrossRef - Metformin use and cardiovascular outcomes in patients with diabetes and chronic kidney disease: a nationwide cohort study
Min Ho Kim, Hyung Jung Oh, Soon Hyo Kwon, Jin Seok Jeon, Hyunjin Noh, Dong Cheol Han, Hyoungnae Kim, Dong-Ryeol Ryu
Kidney Research and Clinical Practice.2021; 40(4): 660. CrossRef - Different Effects of Metformin and A769662 on Sodium Iodate-Induced Cytotoxicity in Retinal Pigment Epithelial Cells: Distinct Actions on Mitochondrial Fission and Respiration
Chi-Ming Chan, Ponarulselvam Sekar, Duen-Yi Huang, Shu-Hao Hsu, Wan-Wan Lin
Antioxidants.2020; 9(11): 1057. CrossRef - Metformin Reduces Lipotoxicity-Induced Meta-Inflammation in β-Cells through the Activation of GPR40-PLC-IP3 Pathway
Ximei Shen, Beibei Fan, Xin Hu, Liufen Luo, Yuanli Yan, Liyong Yang
Journal of Diabetes Research.2019; 2019: 1. CrossRef
- Metformin enhances METTL14-Mediated m6A methylation to alleviate NIT-1 cells apoptosis induced by hydrogen peroxide
- Others
- Rg3 Improves Mitochondrial Function and the Expression of Key Genes Involved in Mitochondrial Biogenesis in C2C12 Myotubes
- Min Joo Kim, Young Do Koo, Min Kim, Soo Lim, Young Joo Park, Sung Soo Chung, Hak C. Jang, Kyong Soo Park
- Diabetes Metab J. 2016;40(5):406-413. Published online August 12, 2016
- DOI: https://doi.org/10.4093/dmj.2016.40.5.406
- 4,923 View
- 71 Download
- 21 Web of Science
- 22 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Panax ginseng has glucose-lowering effects, some of which are associated with the improvement in insulin resistance in skeletal muscle. Because mitochondria play a pivotal role in the insulin resistance of skeletal muscle, we investigated the effects of the ginsenoside Rg3, one of the active components ofP. ginseng , on mitochondrial function and biogenesis in C2C12 myotubes.Methods C2C12 myotubes were treated with Rg3 for 24 hours. Insulin signaling pathway proteins were examined by Western blot. Cellular adenosine triphosphate (ATP) levels and the oxygen consumption rate were measured. The protein or mRNA levels of mitochondrial complexes were evaluated by Western blot and quantitative reverse transcription polymerase chain reaction analysis.
Results Rg3 treatment to C2C12 cells activated the insulin signaling pathway proteins, insulin receptor substrate-1 and Akt. Rg3 increased ATP production and the oxygen consumption rate, suggesting improved mitochondrial function. Rg3 increased the expression of peroxisome proliferator-activated receptor γ coactivator 1α, nuclear respiratory factor 1, and mitochondrial transcription factor, which are transcription factors related to mitochondrial biogenesis. Subsequent increased expression of mitochondrial complex IV and V was also observed.
Conclusion Our results suggest that Rg3 improves mitochondrial function and the expression of key genes involved in mitochondrial biogenesis, leading to an improvement in insulin resistance in skeletal muscle. Rg3 may have the potential to be developed as an anti-hyperglycemic agent.
-
Citations
Citations to this article as recorded by- Comparison of Ginseng Leaf Extract and Its Acid-Treated Form, UG0712 Between Their Effects on Exercise Performance in Mice
Young Jin Lee, Su Hyun Yu, Gwang Yeong Seok, Su Yeon Kim, Mi Jeong Kim, Inhye Jeong, Wan Heo, Bo Su Lee, Seon Gil Do, Bok Kyung Han, Young Jun Kim
Food Supplements and Biomaterials for Health.2024;[Epub] CrossRef - Ginsenosides for the treatment of insulin resistance and diabetes: Therapeutic perspectives and mechanistic insights
Tae Hyun Kim
Journal of Ginseng Research.2024; 48(3): 276. CrossRef - Preparation and bioactivity of the rare ginsenosides Rg3 and Rh2: An updated review
Wenqi Xu, Wei Lyu, Cuicui Duan, Fumin Ma, Xiaolei Li, Dan Li
Fitoterapia.2023; 167: 105514. CrossRef - Ginsenoside Rc, an Active Component of Panax ginseng, Alleviates Oxidative Stress-Induced Muscle Atrophy via Improvement of Mitochondrial Biogenesis
Aeyung Kim, Sang-Min Park, No Soo Kim, Haeseung Lee
Antioxidants.2023; 12(8): 1576. CrossRef - Ginsenoside Rg3 protects glucocorticoid‑induced muscle atrophy in vitro through improving mitochondrial biogenesis and myotube growth
Ryuni Kim, Jee Kim, Sang-Jin Lee, Gyu-Un Bae
Molecular Medicine Reports.2022;[Epub] CrossRef - Beneficial Effects of Walnut Oligopeptides on Muscle Loss in Senescence-Accelerated Mouse Prone-8 (SAMP8) Mice: Focusing on Mitochondrial Function
Rui Fan, Yuntao Hao, Qian Du, Jiawei Kang, Meihong Xu, Yong Li
Nutrients.2022; 14(10): 2051. CrossRef - Ginseng and ginsenosides: Therapeutic potential for sarcopenia
Weiwei Zha, Yuanhai Sun, Wenwen Gong, Linghuan Li, Wonnam Kim, Hanbing Li
Biomedicine & Pharmacotherapy.2022; 156: 113876. CrossRef - Bioactive Oligopeptides from Ginseng (Panax ginseng Meyer) Suppress Oxidative Stress-Induced Senescence in Fibroblasts via NAD+/SIRT1/PGC-1α Signaling Pathway
Na Zhu, Mei-Hong Xu, Yong Li
Nutrients.2022; 14(24): 5289. CrossRef - Review of ginsenosides targeting mitochondrial function to treat multiple disorders: Current status and perspectives
Qingxia Huang, Song Gao, Daqing Zhao, Xiangyan Li
Journal of Ginseng Research.2021; 45(3): 371. CrossRef - The Effects of Korean Red Ginseng on Biological Aging and Antioxidant Capacity in Postmenopausal Women: A Double-Blind Randomized Controlled Study
Tae-Ha Chung, Ji-Hye Kim, So-Young Seol, Yon-Ji Kim, Yong-Jae Lee
Nutrients.2021; 13(9): 3090. CrossRef - A comprehensive review on the phytochemistry, pharmacokinetics, and antidiabetic effect of Ginseng
Yage Liu, Hao Zhang, Xuan Dai, Ruyuan Zhu, Beibei Chen, Bingke Xia, Zimengwei Ye, Dandan Zhao, Sihua Gao, Alexander N. Orekhov, Dongwei Zhang, Lili Wang, Shuzhen Guo
Phytomedicine.2021; 92: 153717. CrossRef - Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes
Federica Zatterale, Michele Longo, Jamal Naderi, Gregory Alexander Raciti, Antonella Desiderio, Claudia Miele, Francesco Beguinot
Frontiers in Physiology.2020;[Epub] CrossRef - Stereoisomer-specific ginsenoside 20(S)-Rg3 reverses replicative senescence of human diploid fibroblasts via Akt-mTOR-Sirtuin signaling
Kyeong-Eun Yang, Hyun-Jin Jang, In-Hu Hwang, Eun Mi Hong, Min-Goo Lee, Soon Lee, Ik-Soon Jang, Jong-Soon Choi
Journal of Ginseng Research.2020; 44(2): 341. CrossRef - Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: Pharmacology and mechanisms
Wenxiang Fan, Yongliang Huang, Hui Zheng, Shuiqin Li, Zhuohong Li, Li Yuan, Xi Cheng, Chengshi He, Jianfeng Sun
Biomedicine & Pharmacotherapy.2020; 132: 110915. CrossRef - Ca2+-activated mitochondrial biogenesis and functions improve stem cell fate in Rg3-treated human mesenchymal stem cells
Taeui Hong, Moon Young Kim, Dat Da Ly, Su Jung Park, Young Woo Eom, Kyu-Sang Park, Soon Koo Baik
Stem Cell Research & Therapy.2020;[Epub] CrossRef - Mitochondrial Dysfunction in Adipocytes as a Primary Cause of Adipose Tissue Inflammation
Chang-Yun Woo, Jung Eun Jang, Seung Eun Lee, Eun Hee Koh, Ki-Up Lee
Diabetes & Metabolism Journal.2019; 43(3): 247. CrossRef - Ginsenoside Rg3 upregulates myotube formation and mitochondrial function, thereby protecting myotube atrophy induced by tumor necrosis factor-alpha
Sang-Jin Lee, Ju Hyun Bae, Hani Lee, Hyunji Lee, Jongsun Park, Jong-Sun Kang, Gyu-Un Bae
Journal of Ethnopharmacology.2019; 242: 112054. CrossRef - Therapeutic Potential of Ginsenosides as an Adjuvant Treatment for Diabetes
Litao Bai, Jialiang Gao, Fan Wei, Jing Zhao, Danwei Wang, Junping Wei
Frontiers in Pharmacology.2018;[Epub] CrossRef - Ginseng and obesity
Zhipeng Li, Geun Eog Ji
Journal of Ginseng Research.2018; 42(1): 1. CrossRef - Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions
Padmanaban Mohanan, Sathiyamoorthy Subramaniyam, Ramya Mathiyalagan, Deok-Chun Yang
Journal of Ginseng Research.2018; 42(2): 123. CrossRef - Inactivation of glycogen synthase kinase-3β (GSK-3β) enhances skeletal muscle oxidative metabolism
W.F. Theeuwes, H.R. Gosker, R.C.J. Langen, K.J.P. Verhees, N.A.M. Pansters, A.M.W.J. Schols, A.H.V. Remels
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2017; 1863(12): 3075. CrossRef - Anti-Fatigue Effects of Small Molecule Oligopeptides Isolated from Panax ginseng C. A. Meyer in Mice
Lei Bao, Xiaxia Cai, Junbo Wang, Yuan Zhang, Bin Sun, Yong Li
Nutrients.2016; 8(12): 807. CrossRef
- Comparison of Ginseng Leaf Extract and Its Acid-Treated Form, UG0712 Between Their Effects on Exercise Performance in Mice
- Effects of Sulfonylureas on Peroxisome Proliferator-Activated Receptor γ Activity and on Glucose Uptake by Thiazolidinediones
- Kyeong Won Lee, Yun Hyi Ku, Min Kim, Byung Yong Ahn, Sung Soo Chung, Kyong Soo Park
- Diabetes Metab J. 2011;35(4):340-347. Published online August 31, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.4.340
- 4,292 View
- 41 Download
- 20 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Sulfonylurea primarily stimulates insulin secretion by binding to its receptor on the pancreatic β-cells. Recent studies have suggested that sulfonylureas induce insulin sensitivity through peroxisome proliferator-activated receptor γ (PPARγ), one of the nuclear receptors. In this study, we investigated the effects of sulfonylurea on PPARγ transcriptional activity and on the glucose uptake via PPARγ.
Methods Transcription reporter assays using Cos7 cells were performed to determine if specific sulfonylureas stimulate PPARγ transactivation. Glimepiride, gliquidone, and glipizide (1 to 500 µM) were used as treatment, and rosiglitazone at 1 and 10 µM was used as a control. The effects of sulfonylurea and rosiglitazone treatments on the transcriptional activity of endogenous PPARγ were observed. In addition, 3T3-L1 adipocytes were treated with rosiglitazone (10 µM), glimepiride (100 µM) or both to verify the effect of glimepiride on rosiglitazone-induced glucose uptake.
Results Sulfonylureas, including glimepiride, gliquidone and glipizide, increased PPARγ transcriptional activity, gliquidone being the most potent PPARγ agonist. However, no additive effects were observed in the presence of rosiglitazone. When rosiglitazone was co-treated with glimepiride, PPARγ transcriptional activity and glucose uptake were reduced compared to those after treatment with rosiglitazone alone. This competitive effect of glimepiride was observed only at high concentrations that are not achieved with clinical doses.
Conclusion Sulfonylureas like glimepiride, gliquidone and glipizide increased the transcriptional activity of PPARγ. Also, glimepiride was able to reduce the effect of rosiglitazone on PPARγ agonistic activity and glucose uptake. However, the competitive effect does not seem to occur at clinically feasible concentrations.
-
Citations
Citations to this article as recorded by- Chitosan-Encapsulated Nano-selenium Targeting TCF7L2, PPARγ, and CAPN10 Genes in Diabetic Rats
Omayma A. R. Abozaid, Sawsan M. El-Sonbaty, Neama M. A. Hamam, Moustafa A. Farrag, Ahmad S. Kodous
Biological Trace Element Research.2023; 201(1): 306. CrossRef - Insights from insulin resistance pathways: Therapeutic approaches against Alzheimer associated diabetes mellitus
Ayesha Fauzi, Ewen Se Thoe, Tang Yin Quan, Adeline Chia Yoke Yin
Journal of Diabetes and its Complications.2023; 37(11): 108629. CrossRef - Novel Sulfonanilide Inhibitors of SHIP2 Enhance Glucose Uptake into Cultured Myotubes
Mika E. A. Berg, Jette-Britt Naams, Laura C. Hautala, Tuomas A. Tolvanen, Jari P. Ahonen, Sanna Lehtonen, Kristiina Wähälä
ACS Omega.2020; 5(3): 1430. CrossRef - Diabetic Theory in Anti-Alzheimer’s Drug Research and Development - Part 1: Therapeutic Potential of Antidiabetic Agents
Agnieszka Jankowska, Anna Wesołowska, Maciej Pawłowski, Grażyna Chłoń-Rzepa
Current Medicinal Chemistry.2020; 27(39): 6658. CrossRef - Moringa concanensis Nimmo extracts ameliorates hyperglycemia-mediated oxidative stress and upregulates PPARγ and GLUT4 gene expression in liver and pancreas of streptozotocin-nicotinamide induced diabetic rats
Brindha Banu Balakrishnan, Kalaivani Krishnasamy, Vijayakumar Mayakrishnan, Arokiyaraj Selvaraj
Biomedicine & Pharmacotherapy.2019; 112: 108688. CrossRef - PPARγ Agonistic Activity of Sulphonylureas
Debjani Banerjee, Harnovdeep Singh Bharaj, Moulinath Banerjee
Endocrine, Metabolic & Immune Disorders - Drug Targets.2019; 19(4): 467. CrossRef - Glimepiride treatment in a patient with type A insulin resistance syndrome due to a novel heterozygous missense mutation in the insulin receptor gene
Zhimin Huang, Juan Liu, Kaka Ng, Xuesi Wan, Lijuan Xu, Xiaoying He, Zhihong Liao, Yanbing Li
Journal of Diabetes Investigation.2018; 9(5): 1075. CrossRef - Arsenic, Cadmium, and Lead Like Troglitazone Trigger PPARγ-Dependent Poly (ADP-Ribose) Polymerase Expression and Subsequent Apoptosis in Rat Brain Astrocytes
Rajesh Kushwaha, Juhi Mishra, Sachin Tripathi, Puneet Khare, Sanghamitra Bandyopadhyay
Molecular Neurobiology.2018; 55(3): 2125. CrossRef - Docosahexaenoic acid up‐regulates both PI3K/AKT‐dependent FABP7–PPARγ interaction and MKP3 that enhance GFAP in developing rat brain astrocytes
Sachin Tripathi, Rajesh Kushwaha, Juhi Mishra, Manoj Kumar Gupta, Harish Kumar, Somali Sanyal, Dhirendra Singh, Sabyasachi Sanyal, Amogh Anant Sahasrabuddhe, Mohan Kamthan, Mohana Krishna Reddy Mudiam, Sanghamitra Bandyopadhyay
Journal of Neurochemistry.2017; 140(1): 96. CrossRef - Antiglycation and cell protective actions of metformin and glipizide in erythrocytes and monocytes
Krishna Adeshara, Rashmi Tupe
Molecular Biology Reports.2016; 43(3): 195. CrossRef - The therapeutic journey of pyridazinone
Wasim Akhtar, M. Shaquiquzzaman, Mymoona Akhter, Garima Verma, Mohemmed Faraz Khan, M. Mumtaz Alam
European Journal of Medicinal Chemistry.2016; 123: 256. CrossRef - Antidiabetic effect of novel benzenesulfonylureas as PPAR-γ agonists and their anticancer effect
Chetna Kharbanda, Mohammad Sarwar Alam, Hinna Hamid, Kalim Javed, Abhijeet Dhulap, Sameena Bano, Yakub Ali
Bioorganic & Medicinal Chemistry Letters.2015; 25(20): 4601. CrossRef - Method Development and Validation of Amlodipine, Gliquidone and Pioglitazone: Application in the Analysis of Human Serum
Agha Zeeshan Mirza, M. Saeed Arayne, Najma Sultana
Analytical Chemistry Letters.2014; 4(5-6): 302. CrossRef - Gliquidone decreases urinary protein by promoting tubular reabsorption in diabetic Goto-Kakizaki rats
Jian-Ting Ke, Mi Li, Shi-Qing Xu, Wen-Jian Zhang, Yong-Wei Jiang, Lan-yun Cheng, Li Chen, Jin-Ning Lou, Wei Wu
Journal of Endocrinology.2014; 220(2): 129. CrossRef - Extension of Drosophila lifespan by cinnamon through a sex-specific dependence on the insulin receptor substrate chico
Samuel E. Schriner, Steven Kuramada, Terry E. Lopez, Stephanie Truong, Andrew Pham, Mahtab Jafari
Experimental Gerontology.2014; 60: 220. CrossRef - Crif1 Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
Min Jeong Ryu, Soung Jung Kim, Yong Kyung Kim, Min Jeong Choi, Surendar Tadi, Min Hee Lee, Seong Eun Lee, Hyo Kyun Chung, Saet Byel Jung, Hyun-Jin Kim, Young Suk Jo, Koon Soon Kim, Sang-Hee Lee, Jin Man Kim, Gi Ryang Kweon, Ki Cheol Park, Jung Uee Lee, Yo
PLoS Genetics.2013; 9(3): e1003356. CrossRef - Glimepiride attenuates Aβ production via suppressing BACE1 activity in cortical neurons
Feiyang Liu, Yijin Wang, Ming Yan, Luyong Zhang, Tao Pang, Hong Liao
Neuroscience Letters.2013; 557: 90. CrossRef - Pharmacologic agents for type 2 diabetes therapy and regulation of adipogenesis
A. Cignarelli, F. Giorgino, R. Vettor
Archives of Physiology and Biochemistry.2013; 119(4): 139. CrossRef - Labisia pumilaUpregulates Peroxisome Proliferator-Activated Receptor Gamma Expression in Rat Adipose Tissues and 3T3-L1 Adipocytes
Fazliana Mansor, Harvest F. Gu, Claes-Göran Östenson, Louise Mannerås-Holm, Elisabet Stener-Victorin, Wan Nazaimoon Wan Mohamud
Advances in Pharmacological Sciences.2013; 2013: 1. CrossRef - Protocol for effective differentiation of 3T3-L1 cells to adipocytes
Katja Zebisch, Valerie Voigt, Martin Wabitsch, Matthias Brandsch
Analytical Biochemistry.2012; 425(1): 88. CrossRef
- Chitosan-Encapsulated Nano-selenium Targeting TCF7L2, PPARγ, and CAPN10 Genes in Diabetic Rats
- Effect of Adipose Differentiation-Related Protein (ADRP) on Glucose Uptake of Skeletal Muscle.
- Yun Hyi Ku, Min Kim, Sena Kim, Ho Seon Park, Han Jong Kim, In Kyu Lee, Dong Hoon Shin, Sung Soo Chung, Sang Gyu Park, Young Min Cho, Hong Kyu Lee, Kyong Soo Park
- Korean Diabetes J. 2009;33(3):206-214. Published online June 1, 2009
- DOI: https://doi.org/10.4093/kdj.2009.33.3.206
- 2,141 View
- 24 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Skeletal muscle is the most important tissue contributing to insulin resistance. Several studies have shown that accumulation of intramyocellular lipid is associated with the development of insulin resistance. Thus, proteins involved in lipid transport, storage and metabolism might also be involved in insulin action in skeletal muscle. Adipose differentiation-related protein (ADRP), which is localized at the surface of lipid droplets, is known to be regulated by peroxisome proliferator activated receptor gamma (PPARgamma). However, it is not known whether ADRP plays a role in regulating glucose uptake and insulin action in skeletal muscle. METHODS: ADRP expression in skeletal muscle was measured by RT-PCR and western blot in db/db mice with and without PPARgamma agonist. The effect of PPARgamma agonist or high lipid concentration (0.4% intralipos) on ADRP expression was also obtained in cultured human skeletal muscle cells. Glucose uptake was measured when ADRP was down-regulated with siRNA or when ADRP was overexpressed with adenovirus. RESULTS: ADRP expression increased in the skeletal muscle of db/db mice in comparison with normal controls and tended to increase with the treatment of PPARgamma agonist. In cultured human skeletal muscle cells, the treatment of PPARgamma agonist or high lipid concentration increased ADRP expression. siADRP treatment decreased both basal and insulin-stimulated glucose uptake whereas ADRP overexpression increased glucose uptake in cultured human skeletal muscle cells. CONCLUSION: ADRP expression in skeletal muscle is increased by PPARgamma agonist or exposure to high lipid concentration. In these conditions, increased ADRP contributed to increase glucose uptake. These results suggest that insulin-sensitizing effects of PPARgamma are at least partially achieved by the increase of ADRP expression, and ADRP has a protective effect against intramyocellular lipid-induced insulin resistance.
- Common Genetic Polymorphisms in the Promoter of Resistin Gene are Major Determinants of Plasma Resistin Concentrations in Humans.
- Young Min Cho, Byung Soo Youn, Sung Soo Chung, Ki Woo Kim, Bo Kyeong Koo, Kang Yeol Yu, Hong Je Park, Hyoung Doo Shin, Hak Chul Jang, Kyong Soo Park, Seong Yeon Kim, Hong Kyu Lee
- Korean Diabetes J. 2004;28(1):9-19. Published online February 1, 2004
- 1,090 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Resistin has been postulated to be an important link between obesity and insulin resistance. Genetic polymorphisms in the resistin gene promotor have been suggested as a determinant of the expression of resistin mRNA, which is possibly associated with obesity and insulin resistance. In this study, the association between the genotype of the resistin promoter, and its plasma concentrations, were investigated. METHODS: The g.-537A>C and g.-420C>G polymorphisms in the resistin promoter were examined, and the levels of plasma resistin measured in the Korean subjects, both with and without type 2 diabetes. Haplotype-based promoter activity and the gel electrophoretic mobility-shift assays(EMSA) were also performed. RESULTS: The -420G and the -537A alleles, which were in linkage disequilibrium, were associated with higher plasma resistin concentrations. Individuals with the A-G(-537 A and -420G) haplotypes showed significantly higher plasma resistin levels than those that did not. The haplotypes A-G had modestly increased promoter activities compared to the other haplotypes. The EMSA revealed the -420 G allele to be specific for binding of the nuclear proteins from adipocytes and monocytes. However, neither polymorphism was associated with type 2 diabetes or obesity in our study subjects. CONCLUSION: Polymorphisms in the promoter of the resistin gene are major determinants of plasma resistin concentrations in humans

 KDA
KDA
 First
First Prev
Prev





